Mind the Gap
Governance at Neuron Speed
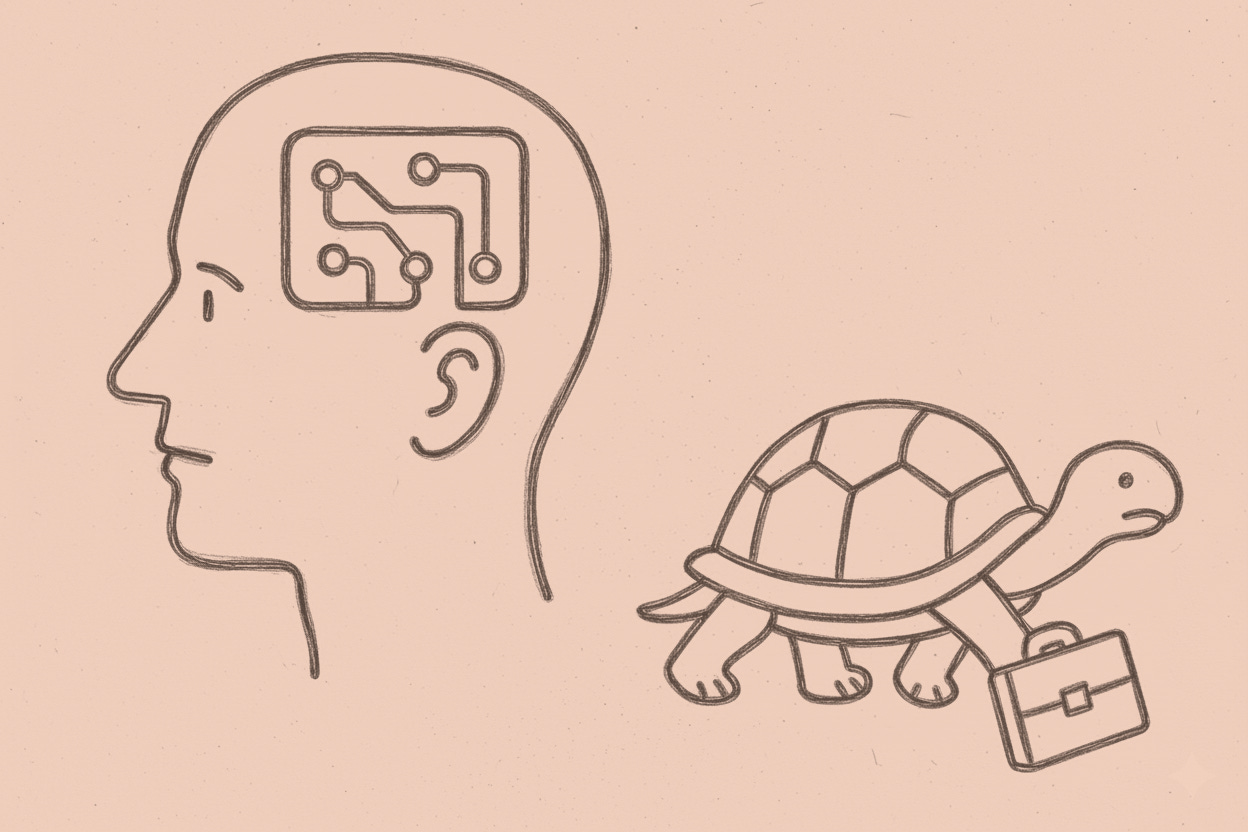
The electrodes are already mapping thoughts while ethics committees are still finding the conference room. Precision Neuroscience received FDA clearance in March 2025. By the time France assembled its eight-person monitoring committee, the devices were translating intention into action. Governance remained under construction. Technology moves at neuron …